Description
Procedures
Information for Patients and Professionals
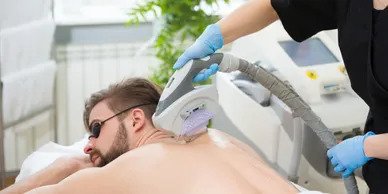


Medical lasers are medical devices that use precisely focused light sources to treat or remove tissues.
The term “laser” stands for light amplification by stimulated emission of radiation. Ordinary light, such as that from a light bulb, has many wavelengths and spreads in all directions. Laser light, on the other hand, has a specific wavelength. It is focused in a narrow beam and creates a very high-intensity light. Because lasers can focus very accurately on tiny areas, they can be used for very precise surgical work or for cutting through tissue (in place of a scalpel).
Lasers are used in many types of surgical procedures.
Some examples include
- Cosmetic surgery (to remove tattoos, scars, stretch marks, sunspots, wrinkles, birthmarks, spider veins or hair)
- Refractive eye surgery (to reshape the cornea in order to correct or improve vision as in LASIK or PRK)
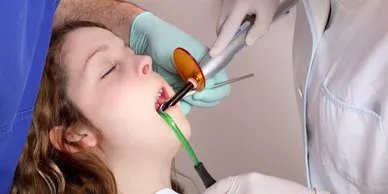
- Dental procedures (such as endodontic/periodontic procedures, tooth whitening, and oral surgery)
- General surgery (such as tumor removal, cataract removal, breast surgery, plastic surgery and most other surgical procedures)
Laws, Regulations & Performance Standards
Required Reports for the Medical Laser Mfg.
Industry Guidance - Documents of Interest


Manufacturers of electronic radiation emitting products sold in the United States are responsible for compliance with the Federal Food, Drug and Cosmetic Act (FFDCA), Chapter V, Subchapter C - Electronic Product Radiation Control. Manufacturers of surgical laser products are responsible for compliance with all applicable requirements of Title 21 Code of Federal Regulations (Subchapter J, Radiological Health) Parts 1000 through 1005:
- 1000 - General
- 1002 - Records and Reports
- 1003 - Notification of defects or failure to comply
- 1004 - Repurchase, repairs, or replacement of electronic products
- 1005 - Importation of electronic products
- 1010 - Performance standards for electronic products: general
- 1040.10 - Lasers and Products Incorporating Lasers
- 1040.11 - Specific Purpose Laser Products
- Federal Register - Laser Products; Proposed Amendment to Performance Standard
- Compliance Guide for Laser Products (FDA 86-8260) (PDF Only) (PDF - 105KB)
- Frequently Asked Questions Regarding the Laser Notice 53 - Guidance for Industry and FDA Staff - Approval of Alternate Means of Labeling for Laser Products (issued March 23, 2007)
- Tabulated Values of Accessible Emission Limits for Laser Products (PDF - 1.7MB) Notices to the Laser Industry
- Quality Control Practices for Compliance with the Federal Laser Product Performance Standard (PDF - 1.1MB)
- Date of Manufacture Label on Radiation-Emitting Consumer Electronics
- Tabulated Values of Accessible Emission Limits for Laser Products (PDF - 1.7MB)
- Guidance for Industry and FDA Staff - Addition of URLs to Electronic Product Labeling
